Learn how to make liquid sculptures from a hand warmer in simple steps. First buy an instant reusable hand warmer which has sodium acetate in it and keep it ready. Now use 4 packets of the hand warmer and stir them out with water. Transfer them to a bottle and keep it aside. Take a sodium acetate crystal from a used hand warmer and place it in a plate. Now pour the liquid slowly on the crystal and you can see the liquid turns solid as you pour it. Design your masterpiece using all the liquid....

Watch this science video tutorial from Nurd Rage on how to make sodium silicate from drain cleaner and gel beads with Dr. Lithium.

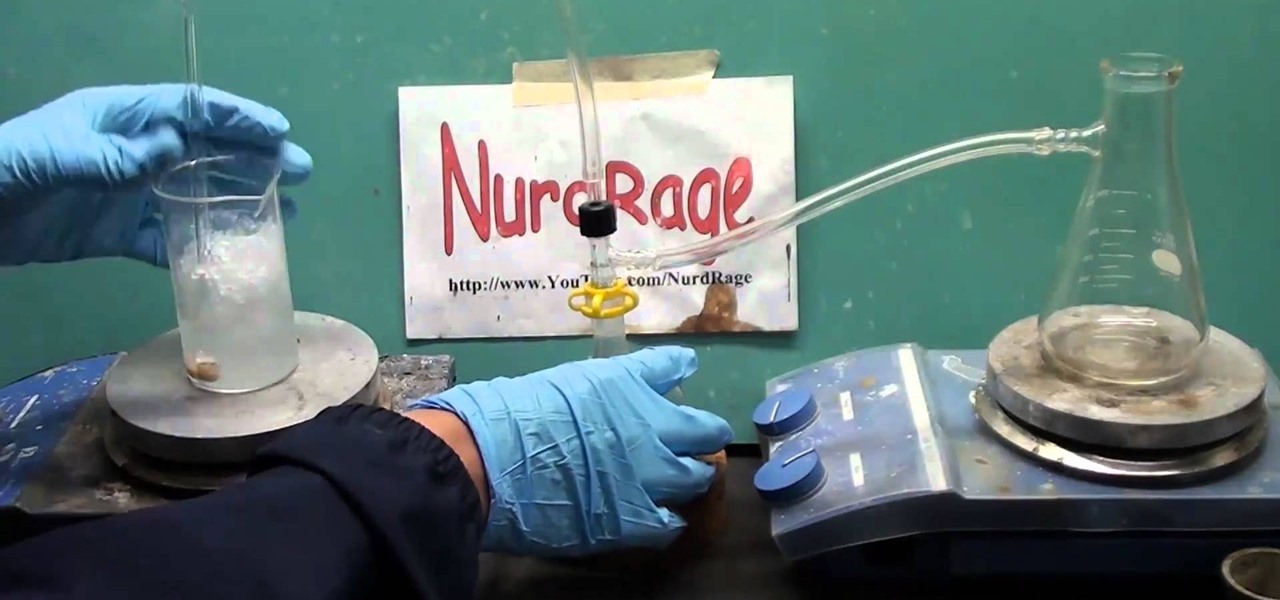
Watch this science video tutorial from Nurd Rage on how to make hot ice with Dr. Lithium. This is the complete guide to making hot ice, more correctly called sodium acetate. See how to create it, fix it, and use it. All methods from baking soda and vinegar to laboratory synthesis are shown.

Sodium (chemical symbol Na) is an interesting element. It reacts in contact with both oxygen and water, and several sodium salts are used to produce a yellow color in fireworks.

This video in the Education category will show you how to make hot ice using Sodium Acetate Trihydrate. For this purpose you will need a pan, 100g of Sodium Acetate Trihydrate, 25ml of water, a wooden spoon and a glass. Take the Sodium Acetate Trihydrate and put 100g in the pan. Then place the pan on a stove and turn to medium heat. The Sodium Acetate Trihydrate will start to melt. In about five minutes when it has melted fully, add 25ml of water. Let the solution simmer for two minutes while...

In this video tutorial, viewers learn how to make "hot ice". Begin by adding water into a pan and heat it until it’s simmering, but not boiling. Add the sodium acetate to the water. Keep adding the sodium acetate until the water cannot dissolve it anymore. Stir constantly. Now pour the solution into a glass or container. Do not pour in any undissolved crystals. Place the solution into the refrigerator for 45 minutes. Now pour the solution into a container. The liquid will instantly turn into ...

Watch this science video tutorial from Nurd Rage on how to make a desiccator bag for drying chemicals with Dr. Lithium.

Unless you're a high-schooler building a nuclear fusion reactor, the hardest part of a science investigatory project often is coming up with a good idea. You want it to be cool yet feasible, novel but still useful.

Watch this amazing video tutorial to learn how to instantly make ice. This is a simple experiment turning a liquid to a solid with just a touch. Just find some sodium acetate and water to start. Boil it, then chill it, then touch it! And in an instant you have ice! If you want to be creative (or you're just bored on a Sunday afternoon) you can pour some ice sculptures to amaze children of all ages. Check out this awesome how-to video and cook up some hot ice.

In this home-science tutorial from WonderHowTo favorite Nurdrage, you'll learn how to create hydrochloric acid using 140 grams of sodium bisulfate, a pH lowering compound available in most pool stores, and 60 grams of sodium chloride salt and an external heat source. Watch for a full demonstration of the process and complete, step-by-step instructions.

In this video tutorial, viewers learn how to do a sodium and water experiment. Sodium is a silver metal that is very reactive. When exposed oxygen in the air, an outer coding of sodium oxide will form. Simply drop a piece of sodium into a cup of water. When dropped in water, sodium reacts to form sodium hydroxide and hydrogen gas. The sodium will constant move around in the water. Sometimes the heated reaction will cause the nitrogen gas to ignite. Under the right condition, it may even cause...

In this video, we learn how to extract sodium to make sodium metal. First place soup can in a pot of water and place the sodium mixture into the can. Place a cap on top and leave it on while the can is cooling off. It should create a tight seal until you will be able to open it. Once you can open it, pour mineral oil on top of the sodium. Filter out this mixture to find the chunks of sodium that is in the mixture. While wearing gloves, take out the sodium and place it into water to see if it ...

If you've ever used a heating pad or hand warmer, you essentially know what "hot ice" is. It's supersaturated sodium acetate, and it's actually fairly easy to make at home out of sodium acetate crystals. You can also make it out of vinegar and baking soda (directions at the bottom of this article).

In this how-to video, you will learn how to make potassium trichromate. You will need potassium chromate and dichromate. It is easy to go between the two. You can add a base to the dichromate to make the chromate. It will go from orange to yellow. The trichromate can be produced. You will need nitric acid, potassium dichromate, and sodium disulphate. First, make a solution of sodium sulphate. Take a long test tube and the potassium dichromate to it. Add double the volume of concentrated nitri...

In this video tutorial, viewers learn how to make "hot ice". Users will need sodium acetate. Begin by putting the sodium acetate into a pan. Add a small amount water to the sodium acetate. Heat the mixture on a stove until the sodium acetate has dissolved. Pour the solution into a container. Do not pour in any undissolved crystals. Put the container into the freezer or refrigerator for a while. When the solution cools down to room temperature, take it out. Touch the sodium acetate and it will...

The Mr.G Show presents how to make "hot ice" more commonly known as sodium acetate in the kitchen. You start with one liter of white vinegar which you place into a sauce pan. You add four table spoons of baking soda to the pan being very careful because both chemical together will react an may cause a big mess so be prepared to clean up any boil over that may arise. Wait while this mixture boils down for the real fun to starts. The hot ice created will transform from a liquid to a solid befor...

When a rod mounted in a hand drill is dipped into a liquid and rotated, for certain non-Newtonian fluids the liquid will climb the rod - sometimes to quite spectacular heights.

Its a flavor explosion! Take some cilantro, add in a little Calcium Chloride and Sodium Alginate, and give some crab cakes a new twist! The pearl is one of the most useful treats of Molecular Gastronomy. Learn how to make corn cake with bbq crab and cilantro pearls.

Need to cut back on the sodium? Limiting your daily salt intake can help prevent major diseases. Here's how to cut the sodium out from your diet.

Watch this science video tutorial from Nurd Rage on how to dissolve glass with drain cleaner. They show you how to dissolve that glass with sodium hydroxide (drain cleaner).

This free video science lesson from the Home Scientist demonstrates a simple technique for creating a color-changing solution with sodium permanganate, sodium hydroxide and sugar. For all of the relevant details and detailed, step-by-step instructions, as well as to get started trying this experiment yourself, watch this home-science how-to.

In our personal experience, the hardest part about a science investigatory project is simply coming up with a good idea. And we suggest that for your investigatory project you find a topic that's both novel and useful.

Put pre-mix into bowl. Add whole mustard seed, coriander, fresh ground pepper, and liquid smoke for flavor. You can also add half a teaspoon of hot pepper mix to make it spicy. For meat, use 1/3 ground chuck and 2/3 hamburger; some fat is necessary for a good sausage. Next, add the sodium nitrates, spices and 1 ounce per pound of meat of water into the chuck and hamburger and mix it with your hands. Continue mixing until the meat gets "tacky". Then, the meat needs to be ground and put into th...

Ever wondered how science could turn out to be fun? Here's how: take a piece of paper with a mixture of sodium nitrate and sugar. When heated, sodium nitrate releases oxygen and burns sugar. Sugar alone cannot burn fast. If you want faster results, then take sodium nitrate with sulphur and some reactive metals. Add some coal to it for a faster result. This will help you burn some useless pile in quick time.

Eel sauce, or kabayaki sauce, is a thick, sweet Japanese sauce commonly served as a dipping sauce for Japanese seafood dishes. This eel sauce recipe is quick and easy... as well as delicious.

Hydrazine sulfate has many uses, but most notably, it's been used under the trade name of Sehydrin, a treatment for anorexia, cachexia and some even think cancer. But for we DIY chemists, it's useful for something entirely different— as a substitute for the more dangerous pure liquid hydrazine in chemical reactions. NurdRage shows you how to make it via some hypochlorite and the Ketazine process.

Bottled water is a rip-off. Not only is it pretty much the same stuff that comes out of your tap for free, but plastic bottles are rarely recycled and thus account for a huge amount of the waste that's overflowing our landfills. Next Up: Water Bottles You Can Eat

Want to make boring old colorless water brighten up on command? Well, you can control the color of water with this little magic trick. Actually, it's not really magic, but a classic science experiment known commonly as the iodine clock reaction, which uses the reactions between water and chemicals to instantly colorize water, seemingly by command. You can use different colorless chemicals to produce different colors, and you can even make the color vanish to make the water clear again.

Chlorine gas is a very useful oxidant, which was first introduced as a toxic weapon by the German Army. Even today, it's still used as a weapon, most recently in the Iraq War by insurgents. But chlorine gas has more useful (and less lethal) applications, and if you want to learn how to make some at home, NurdRage has the answers.

The war on dehydration is a commercially burgeoning marketplace. An increasingly sophisticated consumer population hoping to conquer everything from 26-mile marathons to vodka shots is deconstructing every functional remedy in the fight to quell the effects of severe dehydration.

I have a weird fondness for the texture, if not the taste of Velveeta (and Kraft American cheese slices). No other cheese has quite the same amount of slip or smoothness and manages to stay that way, undoubtedly because Velveeta contains sodium alginate, an algae derivative that helps it stay so silky-smooth even as it heats up. It also contains a high level of protein-to-fat ratios, which is what makes it a champion melter.

This free video science lesson from the Home Scientist demonstrates a simple technique for comparing the radioactivity of lithium and sodium. For all of the relevant details and detailed, step-by-step instructions, as well as to get started trying this experiment yourself, watch this home-science how-to.

Salt seems to sneak in everywhere, especially for big meals like Thanksgiving, when it seems every dish calls for salt. Some ideas for cutting down on salt is to cook with unsalted butter, low-sodium broths or use salt substitutes when cooking.

Brew laundry detergent that cleans your clothes for just three cents a load -- a fraction of what it costs with store-bought detergent.

In this how to video, learn to make sodium acetate (hot ice) with household items. Make a heating pad, that is re-usable more than 100 times, with this step by step science experiment.

This is a science experiment illustrating the reaction between chlorine, sodium & water.

This video tutorial is in the Education category which will show you how to make sodium bromate and potassium meta-periodate. This experiment uses toxic chemicals and releases harmful gases. First you have to make a saturated sodium bromide. To this solution then add a small amount of potassium dichromate. The solution now becomes concentrated. Then make the circuit as shown in the video. You need a 3 - 5 ohm resistor in order to reduce the current. Then let the cell run for around 6 hours. S...

This actions is a video tutorial in the Education category where you are going to learn how to synthesize copper(II) carbonate & sodium bicarbonate. For this you will need copper sulphate which is available in root kill and sodium bicarb which is baking soda. Take 100g of copper sulphate and dissolve in about 400ml of water. Now take 69.27g of baking soda. Add baking soda very slowly and keep stirring the solution. You got to be very careful as the chemical reaction will produce lot of carbon...

In this tutorial, we learn how to make silicate salt. First, you will need to add sodium silicate with boiling water. Keep adding the sodium and then stirring in small amounts until it is completely dissolved. Then, allow the mixture to cool off and add in nickel chloride. You will now start to see a spongy green mixture coming from the chloride which is the nickel chloride reacting with the sodium. Do this again with cobalt chloride and watch the crystals start to form. Watch these grow and ...

Two college students; Kevin Martin and Joey Smokey introduce the concept of Molar Mass. They start of by explaining what molar mass is, which is the relationship of a mole and a gram, it totals up the weight(in g)of a molecule. An example: say you have this compound, Sodium phosphate (Na3PO4). You know you have three sodium atoms, one phosphorus atom, and four oxygen atoms. You basically find the weight of each atom, if you have three sodium atoms, you multiply it's atomic mass by 3 (the numb...