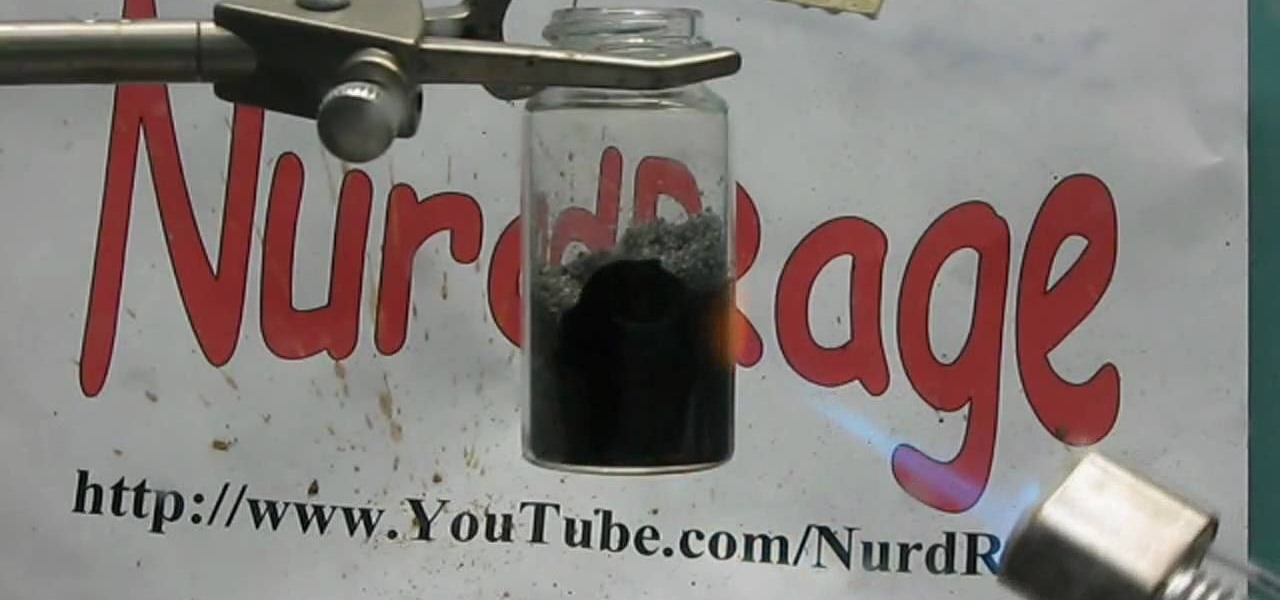
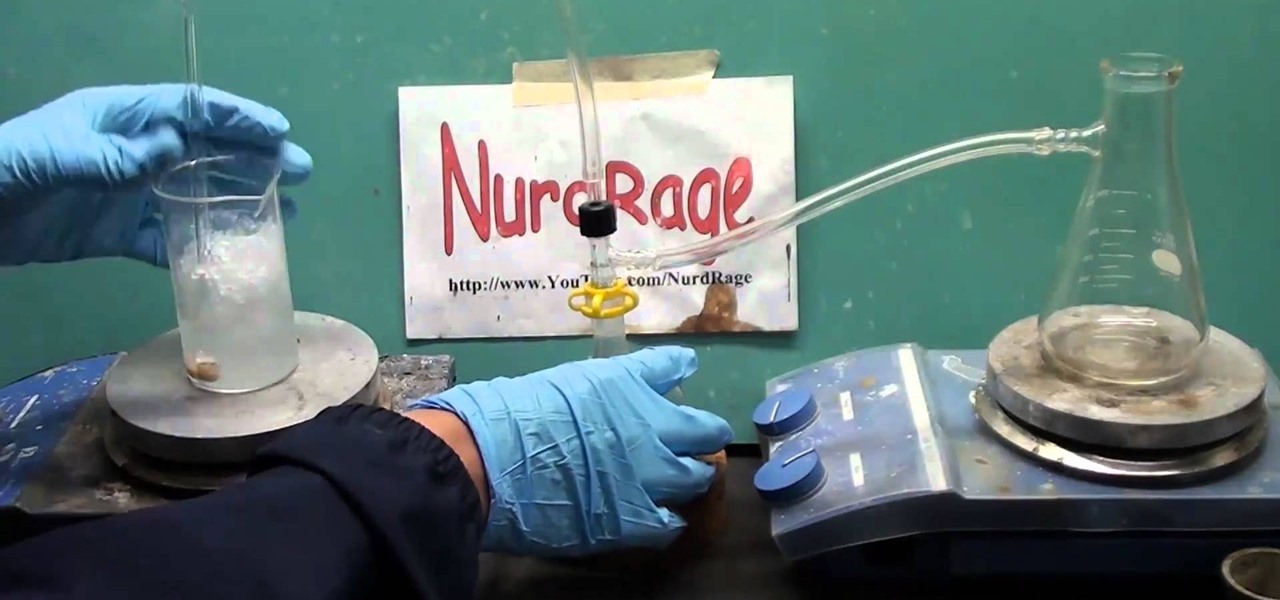
This actions is a video tutorial in the Education category where you are going to learn how to synthesize copper(II) carbonate & sodium bicarbonate. For this you will need copper sulphate which is available in root kill and sodium bicarb which is baking soda. Take 100g of copper sulphate and dissolve in about 400ml of water. Now take 69.27g of baking soda. Add baking soda very slowly and keep stirring the solution. You got to be very careful as the chemical reaction will produce lot of carbon...

Science experiment with acetic acid and sodium bicarbonate (baking soda and vinegar) giving off carbon dioxide and other gases. You will need vinegar, baking soda, a jar and a latex glove. Experiment with acedic acid and sodium bicarbonate.

Brew laundry detergent that cleans your clothes for just three cents a load -- a fraction of what it costs with store-bought detergent.

Electrolytes are solutions containing ions usually found in sports drinks that help maintain proper hydration during extreme physical activity. A balance of electrolytes is important for the normal functioning of the cells and organs of our body. The most popular electrolytes are sodium, potassium, chloride and bicarbonate. Try these tips for buying foods rich in electrolytes in this nutrition how-to video.

In this video, I'll be showing you how classic black snakes work and how to make them at home. There are actually two methods covered in the video — one that uses fire and one that does not. So just choose the one that fits best for your situation.

Watch as Manjula prepares a popular Indian side dish. Dhokla can be made several different ways, here we see how to make it with semolina flour.

Certain ingredients that a professional chef might work with in a restaurant-style setting may seem bizarre, dangerous, and even downright scary to a home cook, and for good reason.

Watch this science video tutorial from Nurd Rage on how to make sodium silicate from drain cleaner and gel beads with Dr. Lithium.

Watch this science video tutorial from Nurd Rage on how to make potassium permanganate with Dr. Lithium with potassium nitrate and manganese dioxide.

In this home-science tutorial from WonderHowTo favorite Nurdrage, you'll learn how to create hydrochloric acid using 140 grams of sodium bisulfate, a pH lowering compound available in most pool stores, and 60 grams of sodium chloride salt and an external heat source. Watch for a full demonstration of the process and complete, step-by-step instructions.

In this video tutorial, viewers learn how to do a sodium and water experiment. Sodium is a silver metal that is very reactive. When exposed oxygen in the air, an outer coding of sodium oxide will form. Simply drop a piece of sodium into a cup of water. When dropped in water, sodium reacts to form sodium hydroxide and hydrogen gas. The sodium will constant move around in the water. Sometimes the heated reaction will cause the nitrogen gas to ignite. Under the right condition, it may even cause...

In this video, we learn how to extract sodium to make sodium metal. First place soup can in a pot of water and place the sodium mixture into the can. Place a cap on top and leave it on while the can is cooling off. It should create a tight seal until you will be able to open it. Once you can open it, pour mineral oil on top of the sodium. Filter out this mixture to find the chunks of sodium that is in the mixture. While wearing gloves, take out the sodium and place it into water to see if it ...

If you've ever used a heating pad or hand warmer, you essentially know what "hot ice" is. It's supersaturated sodium acetate, and it's actually fairly easy to make at home out of sodium acetate crystals. You can also make it out of vinegar and baking soda (directions at the bottom of this article).

In this video tutorial, viewers learn how to make "hot ice". Users will need sodium acetate. Begin by putting the sodium acetate into a pan. Add a small amount water to the sodium acetate. Heat the mixture on a stove until the sodium acetate has dissolved. Pour the solution into a container. Do not pour in any undissolved crystals. Put the container into the freezer or refrigerator for a while. When the solution cools down to room temperature, take it out. Touch the sodium acetate and it will...

Need to cut back on the sodium? Limiting your daily salt intake can help prevent major diseases. Here's how to cut the sodium out from your diet.

Watch this science video tutorial from Nurd Rage on how to make hot ice with Dr. Lithium. This is the complete guide to making hot ice, more correctly called sodium acetate. See how to create it, fix it, and use it. All methods from baking soda and vinegar to laboratory synthesis are shown.

Watch this science video tutorial from Nurd Rage on how to dissolve glass with drain cleaner. They show you how to dissolve that glass with sodium hydroxide (drain cleaner).

Its a flavor explosion! Take some cilantro, add in a little Calcium Chloride and Sodium Alginate, and give some crab cakes a new twist! The pearl is one of the most useful treats of Molecular Gastronomy. Learn how to make corn cake with bbq crab and cilantro pearls.

This free video science lesson from the Home Scientist demonstrates a simple technique for creating a color-changing solution with sodium permanganate, sodium hydroxide and sugar. For all of the relevant details and detailed, step-by-step instructions, as well as to get started trying this experiment yourself, watch this home-science how-to.

Ever wondered how science could turn out to be fun? Here's how: take a piece of paper with a mixture of sodium nitrate and sugar. When heated, sodium nitrate releases oxygen and burns sugar. Sugar alone cannot burn fast. If you want faster results, then take sodium nitrate with sulphur and some reactive metals. Add some coal to it for a faster result. This will help you burn some useless pile in quick time.

All you need is soda bicarbonate or baking soda & a half bottle of ketchup. Use this as inspiration for one of your April Fools Day pranks!

Sodium (chemical symbol Na) is an interesting element. It reacts in contact with both oxygen and water, and several sodium salts are used to produce a yellow color in fireworks.

Chlorine gas is a very useful oxidant, which was first introduced as a toxic weapon by the German Army. Even today, it's still used as a weapon, most recently in the Iraq War by insurgents. But chlorine gas has more useful (and less lethal) applications, and if you want to learn how to make some at home, NurdRage has the answers.

This video in the Education category will show you how to make hot ice using Sodium Acetate Trihydrate. For this purpose you will need a pan, 100g of Sodium Acetate Trihydrate, 25ml of water, a wooden spoon and a glass. Take the Sodium Acetate Trihydrate and put 100g in the pan. Then place the pan on a stove and turn to medium heat. The Sodium Acetate Trihydrate will start to melt. In about five minutes when it has melted fully, add 25ml of water. Let the solution simmer for two minutes while...

I have a weird fondness for the texture, if not the taste of Velveeta (and Kraft American cheese slices). No other cheese has quite the same amount of slip or smoothness and manages to stay that way, undoubtedly because Velveeta contains sodium alginate, an algae derivative that helps it stay so silky-smooth even as it heats up. It also contains a high level of protein-to-fat ratios, which is what makes it a champion melter.

A fire snake, also referred to as a black snake or sugar snake, is a classic science experiment you can do right in your own kitchen using a baking soda and sugar mixture and a fuel to ignite the reaction.

Baking powder and baking soda are two staples almost everyone has around that seem to last forever. But a lot of people don't know that they eventually start to lose their potency after enough time on the shelf. If you can't remember when you bought it, it's probably time for a new box.

This free video science lesson from the Home Scientist demonstrates a simple technique for comparing the radioactivity of lithium and sodium. For all of the relevant details and detailed, step-by-step instructions, as well as to get started trying this experiment yourself, watch this home-science how-to.

This video tutorial is in the Education category which will show you how to make sodium bromate and potassium meta-periodate. This experiment uses toxic chemicals and releases harmful gases. First you have to make a saturated sodium bromide. To this solution then add a small amount of potassium dichromate. The solution now becomes concentrated. Then make the circuit as shown in the video. You need a 3 - 5 ohm resistor in order to reduce the current. Then let the cell run for around 6 hours. S...

In this tutorial, we learn how to make silicate salt. First, you will need to add sodium silicate with boiling water. Keep adding the sodium and then stirring in small amounts until it is completely dissolved. Then, allow the mixture to cool off and add in nickel chloride. You will now start to see a spongy green mixture coming from the chloride which is the nickel chloride reacting with the sodium. Do this again with cobalt chloride and watch the crystals start to form. Watch these grow and ...

Salt seems to sneak in everywhere, especially for big meals like Thanksgiving, when it seems every dish calls for salt. Some ideas for cutting down on salt is to cook with unsalted butter, low-sodium broths or use salt substitutes when cooking.

Two college students; Kevin Martin and Joey Smokey introduce the concept of Molar Mass. They start of by explaining what molar mass is, which is the relationship of a mole and a gram, it totals up the weight(in g)of a molecule. An example: say you have this compound, Sodium phosphate (Na3PO4). You know you have three sodium atoms, one phosphorus atom, and four oxygen atoms. You basically find the weight of each atom, if you have three sodium atoms, you multiply it's atomic mass by 3 (the numb...

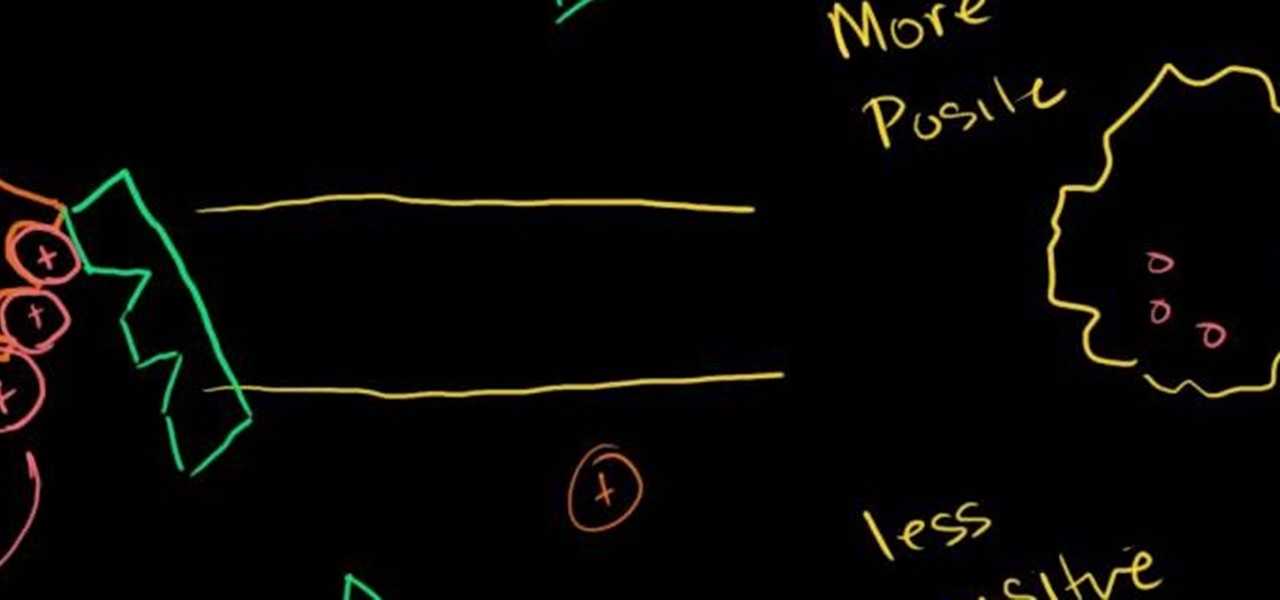
This is a great video presentation of how Sodium Potassium Pump can maintain a voltage gradient across a cell. It also discusses various things like the differences between positive and negative charges and positive and less positive charges etc. The video tries to explain a lot of things happen within a cell when you fluctuate the voltage of sodium as well as positive and negative ions within a cell. You must watch it yourself to see the changes happen within a cell when you make some changes.

In this how to video, learn to make sodium acetate (hot ice) with household items. Make a heating pad, that is re-usable more than 100 times, with this step by step science experiment.

This is a science experiment illustrating the reaction between chlorine, sodium & water.

Learn how to make liquid sculptures from a hand warmer in simple steps. First buy an instant reusable hand warmer which has sodium acetate in it and keep it ready. Now use 4 packets of the hand warmer and stir them out with water. Transfer them to a bottle and keep it aside. Take a sodium acetate crystal from a used hand warmer and place it in a plate. Now pour the liquid slowly on the crystal and you can see the liquid turns solid as you pour it. Design your masterpiece using all the liquid....

Watch this science video tutorial from Nurd Rage on how to make silver different colors by electrochemical anodizing. Without using paint, you can give a silver surface various colors by anodizing it.

If you prefer glow sticks over candles during a power outage, then this how-to is for you! Although glow sticks are used as temporary light sources, there are other applications for them. Divers use them for night diving, fisherman use them to catch swordfish, and the military uses them for light markers, along with infrared versions used in conjunction with night vision devices. But with all these handy uses for glow sticks, the most popular is — recreational use, like dancing at raves, some...

Watch this science video tutorial from Nurd Rage on how to make a desiccator bag for drying chemicals with Dr. Lithium.

Stop serving a scoop of ice cream and put some effort into your next dessert dish! Steal this recipe from Sue McMahon, cookery editor at Woman's Weekly, for a delicious chocolate ruffle cake. To make this ruffle cake you will need about two-and-a-half hours and the following ingredients: