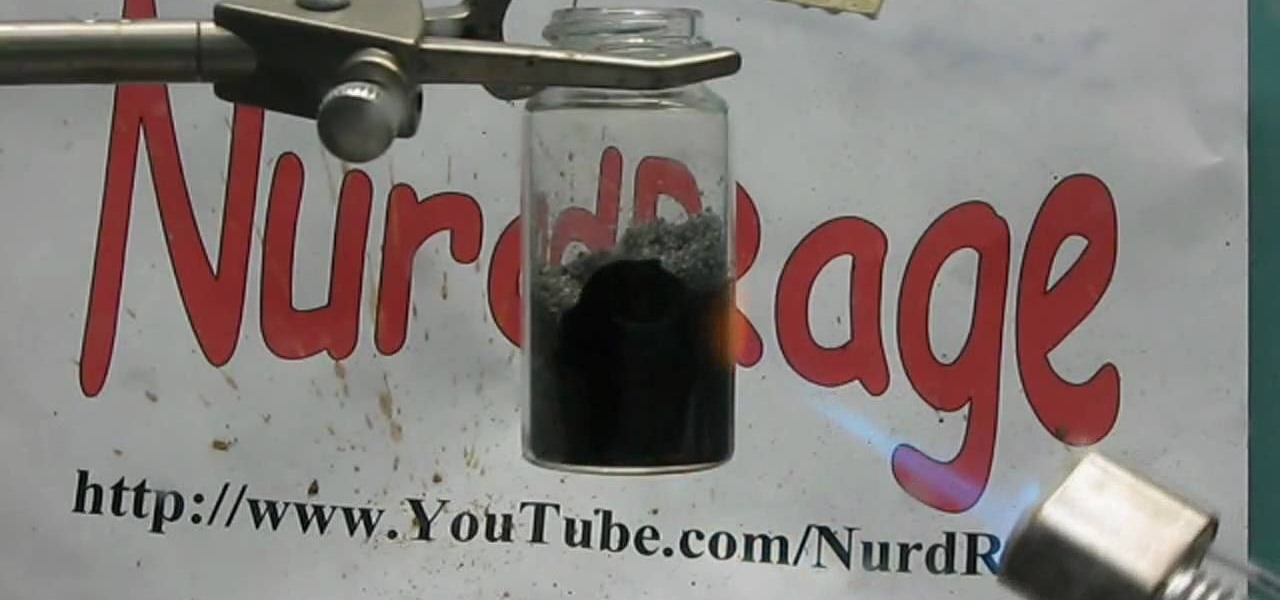
In order to make manganese dioxide electrodes, you're going to need cobalt nitrate and manganese nitrate to do it. Making cobalt nitrate is fairly easy, but making the manganese nitrate is a little more complicated. But not impossible.

What is MnSO4 and MNO2, anyway? They are they molecular formula for Manganese Sulfate and Manganese Dioxide. And you can make one from the other. But how?

This video speaks to everyone who has ever bought anything online, or in fact, anyone who has ever bought anything period. How do you know what you're getting is genuine? Is it a fake product? Is it stolen goods? Is it impure?

If you had to answer the following statement, which answer would you choose? Water is:

You already know how to make sulfuric acid with the metabisulfite and oxidizer method and you saw how to make copper sulfate from copper and sulfuric acid, so now try making sulfuric acid with these two in mind… with sulfuric acid by electrolysis of copper using an inert anode.

It's a stormy winter night, and you're electricity goes out. You could grab some candles to add a little light to your life, or you could use glow-in-the-dark chemicals for a cool luminescent.

There's a few reasons why you might want to purify chemicals by recrystallization, but the best one could be luminescence glow-in-the-dark dust.

Aluminum nitrate nonahydrate is a crystalline hydrate - a salt of aluminum and nitric acid - Al(NO3)3·9H2O. It's used for a variety of things such as antiperspirants, corrosion inhibitors, and petroleum refining, or… glow-in-the-dark powder. Watch this science video tutorial from Nurd Rage on how to make aluminum nitrate nonahydrate with Dr. Lithium.

This is chemistry at its best! Europium is the chemical element (Eu) which was named after Europe. Dysprosium (Dy) is a rare earth element of a metallic silver luster. Watch this science video tutorial from Nurd Rage on how to make europium and dysprosium nitrate salts with Dr. Lithium.

Watch this science video tutorial from Nurd Rage on how to make a desiccator bag for drying chemicals with Dr. Lithium.

Watch this science video tutorial from Nurd Rage on how to make a 100 foot glow stick with Dr. Lithium.

Watch this science video tutorial from Nurd Rage on how to make copper sulfate from copper and sulfuric acid in three ways. They show you how to make copper sulfate from copper and sulfuric acid using two chemical methods and one electrochemical method.

Watch this science video tutorial from Nurd Rage on how to get lithium metal from an Energizer battery. They show you how to get Lithium Metal from an Energizer Ultimate Lithium battery.

Watch this science video tutorial from Nurd Rage on how to make nitric acid. They show three ways to make nitric acid based on two different chemical approaches, both of which can be done using easily accessible materials.

Watch this science video tutorial from Nurd Rage on how to make fire 4 ways without matches by using chemistry, without matches or lighters.

Watch this science video tutorial from Nurd Rage on how to test if a fertilizer has nitrates rather than urea or ammonia as its nitrogen source.

One of the most famous and repeated chemistry experiments involves money. Some would say this is more of a trick than an experiment, but you can be the judge of that. No one can just turn pennies into silver or gold coins, but someone with a few chemicals can. So, if you want to cooler cents in your pocket, try out this chemistry trick yourself. Nurd Rage (Dr. Lithium) shows you how to turn pennies into silver and gold coins using zinc.

Watch this science video tutorial from Nurd Rage on how to make potassium permanganate with Dr. Lithium with potassium nitrate and manganese dioxide.

Watch this science video tutorial from Nurd Rage on how to find chemicals for science experiments with Dr. Lithium.

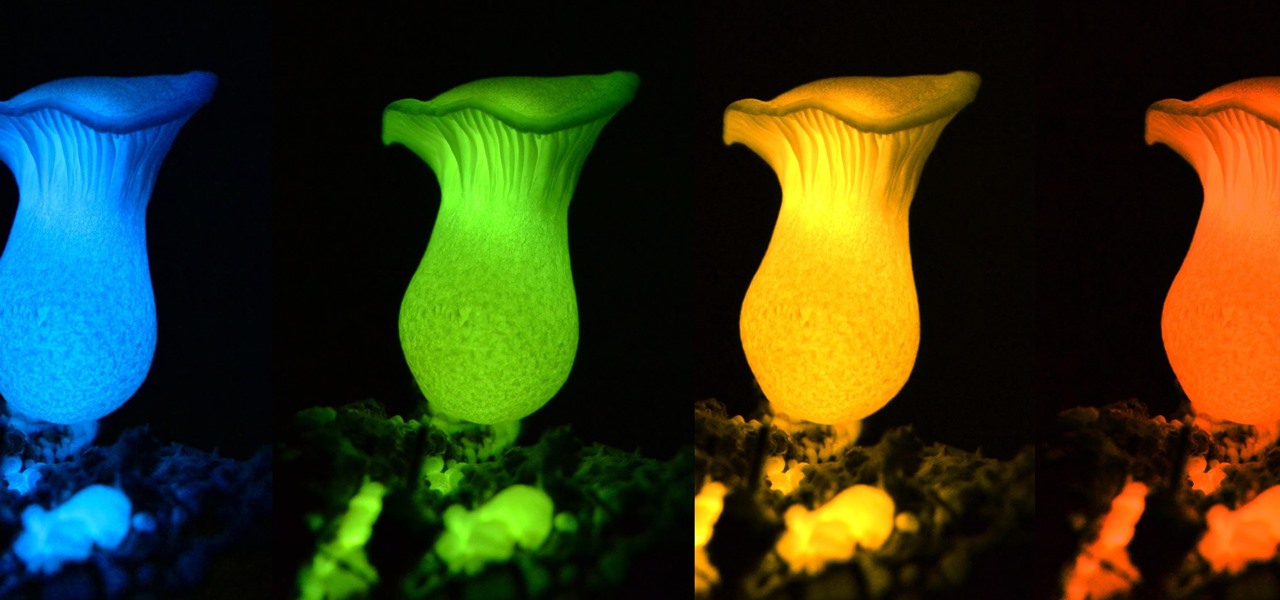
Making a blue glowing substance is easy, as long as you already know the secret to making green luminescent phosphorescent glow powder. By watching the green glow-in-the-dark procedure, you know that you will need strontium nitrate, aluminum nitrate, europium and dysprosium nitrates, and nitric acid. That will make a green glow, but if you want to make a blue version, it's a relatively easy fix...

If you want to make some electrodes for electrochemistry, titanium strips are the way to go. It's rather interesting metal, and it's really hard, but can be cut with standard metalworking tools. Titanium filings are highly flammable, just like iron filings. But not everything is what it seems… this quick video on titanium and passivation electrochemistry tells why it makes it a bad anode if used without further treatment.

How many times have you tried to set fire to candy canes, only to watch in sad frustration as they melt into sticky blobs. We've all been there, right?

Watch this science video tutorial from Nurd Rage on how to compare regular ice with liquid nitrogen-cooled ice with Dr. Lithium.

Watch this science video tutorial from Nurd Rage on how to make iodine from an alkali metal iodide, hydrochloric acid (HCI), and hydroxide peroxide (H2O2).

Ever wonder how to measure the height of a building, without using a tape measure? Well, this crazy science experiment will show you how. Just watch and you'll see how to measure the height of a building with a CRT monitor, gravity and math.

Smash glow? What the heck is that? That's exactly what you'll find out… watch this science video tutorial from Nurd Rage on how to make smash-glow crystals (triboluminescent crystals) with Dr. Lithium.

Watch this science video tutorial from Nurd Rage on how to make a lithium thionyl chloride battery, which is capable of generating 2.8v with enough current to power a LED.

Watch this science video tutorial from Nurd Rage on how to make iodine from sulfuric acid and alkali metal iodide. This is the best way to make elemental iodine from sulfuric acid and sodium or potassium iodide.

Watch this science video tutorial from Nurd Rage on how to make a chemiluminescent reaction with home chemicals. Make a chemiluminescent singlet oxygen red light pulse from two simple chemicals almost anyone can buy: pool chlorine and hydrogen peroxide.

Watch this science video tutorial from Nurd Rage on how to make a complete refillable glow stick. You can make this complete and refillable glow stick with a steampunk-style to it.

Watch this science video tutorial from Nurd Rage on how to restore silver with electrochemistry. You can restore old silver with aluminum foil or a battery by simple electrochemistry.

Watch this science video tutorial from Nurd Rage on how to make silver different colors by electrochemical anodizing. Without using paint, you can give a silver surface various colors by anodizing it.

Watch this science video tutorial from Nurd Rage on how to make silver nitrate from silver and nitric acid. They show the chemistry of making this cool chemistry, colorless solid.

Watch this science video tutorial from Nurd Rage on how to make silver chloride for a photochemistry test. They show the chemistry of photography using silver chloride that they make themselves from table salt and silver nitrate.

Watch this science video tutorial from Nurd Rage on how to make a mirror silvering solution from silver nitrate, ammonia, sugar, and sodium hydroxide.

Watch this science video tutorial from Nurd Rage on how to make a glow stick reaction with real chemicals.

Bioluminescence — the ability of an organism to produce and emit light — is nature's light show. Plants, insects, fish, and bacteria do it, and scientists understand how. Until now, though, we didn't know how fungi glow.

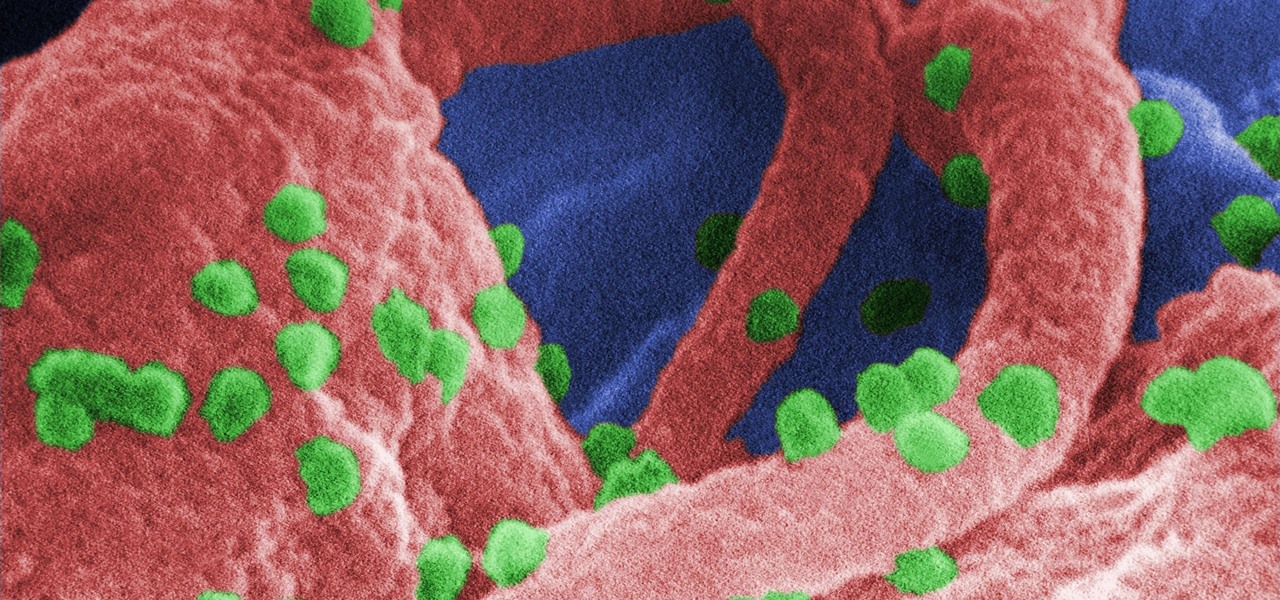
The problem with HIV is that it attacks and kills the very cells of the immune system that are supposed to protect us from infections — white blood cells. But a new technique, developed by scientists at The Scripps Research Institute (TSRI) in La Jolla, California, offers a distinct HIV-killing advantage.

Watch this science video tutorial from Nurd Rage on how to make copper sulfate and zinc batteries. They show you how to make the classic copper sulfate and zinc battery using the incredibly easy "gravity" battery design approach. Great for science fairs and similar projects this battery can be used to explore many basic concepts in batteries.

Attention aspiring scientists and chemists! Learning to balance equations and finding it to be a bit rough? This video is here to help. Learn the basics of chemistry with help from this video on how to balance equations with C (Carbon), H (Hydrogen), and O2 (Oxygen).