
How To: Make a 100 foot glow stick with Dr. Lithium
Watch this science video tutorial from Nurd Rage on how to make a 100 foot glow stick with Dr. Lithium.


Watch this science video tutorial from Nurd Rage on how to make a 100 foot glow stick with Dr. Lithium.

How to make TCPO or bis(2,4,6-trichlorophenyl) oxalate, used in glow stick reactions. WARNING: This procedure should only be performed by, or under the direct supervision of, an experienced chemist. Please refer to the material safety data sheets of all chemicals for their hazards. Synthesis must be performed in a fumehood.

In this video, you will learn how to draw the Lewis Dot structure for CH3CH2OH, or drinking alcohol. To draw the first type of alcohol, draw H, C, C, O, and H horizontally. Now draw, an H above and below both C. Draw the dot structures in between each letter. Hydrogen has one valance electrons, Carbon has four, and Oxygen has six. Now, connect the Lewis Dot Structures. If you are given C2H6O, write down C, O, and C horizontally. Draw a line between the O and the C's. The Carbons will have thr...

This video helps us understand the organic molecules and elimination reaction. Take some sugar in a beaker. Sugar has 12 carbon atoms, 22 hydrogen atoms and 11 atoms of oxygen. The sulphuric acid is poured into the sugar and the color change is observed. The color of the sugar gradually changes into black. The sulphuric acid causes an exothermic reaction which releases a large amount of sulphur dioxide gas. All the water (containing hydrogen and oxygen atoms) is eliminated out of the sugar du...

Try out this science experiment... a classy chemical demonstration. Watch this video tutorial to learn how to make a foamy elephant toothpaste chemical reaction. There's a reason why they call this elephants toothpaste.

Find out how everything in a chemistry lab works, from pipettes to burners to recrystallization to storage. You'll get precise instructions on how to work and perform certain scientific duties in the chem lab, whether it's chemical or just ordinary high school science.

Watch this science video tutorial from Nurd Rage on how to make a chemiluminescent reaction with home chemicals. Make a chemiluminescent singlet oxygen red light pulse from two simple chemicals almost anyone can buy: pool chlorine and hydrogen peroxide.

Watch this science video tutorial from Nurd Rage on how to make a glow stick reaction with real chemicals.

Concerned about the unpronounceable chemicals you see on tubes of toothpaste? Clean your tetth and keep your breath fresh without having to introduce unpleasant chemicals to your system! All you need is some baking soda, hydrogen peroxide, essential oils (mints are best) and a few drops of stevia (optional). Combine together, and smile! You have homemade toothpaste!

A step-by-step how-to video from grooveshark.com explaining how to make your own circuit board or PCB. It's a simple, but long process for copper etching a printed circuit board, so pay attention and pause when needed.

Curious about reduction & oxidation? In this two-part episode of The Science Catalyst hosted by Barry Lambson, the subject of Chemistry, reduction & oxidation is gone over with iron powder and copper sulphate. Oxidation and reduction are heavily related which is why they are both considered "redox reactions". Acids and bases may be thought of as reactions involving hydrogen, or protons, while redox reactions tend to be concerned with electron gain and loss.

Watch this science video tutorial from Nurd Rage on how to make iodine from an alkali metal iodide, hydrochloric acid (HCI), and hydroxide peroxide (H2O2).

Watch this science video tutorial from Nurd Rage on how to make iodine from sulfuric acid and alkali metal iodide. This is the best way to make elemental iodine from sulfuric acid and sodium or potassium iodide.

Watch this science video tutorial from Nurd Rage on how to make a complete refillable glow stick. You can make this complete and refillable glow stick with a steampunk-style to it.

In this video the author shows how to make a never ending foam snake. He starts by speaking about how hydrogen peroxide can be fun. He starts with the requirements first which are a dish soap, a bottle of hydrogen peroxide, some dry yeast, and a red fruit color. Now he fills up a cup with hydrogen peroxide, adds the color and two table spoons of dish soap. Now he shows how to use yeast which is used to remove the oxygen from the hydrogen peroxide. And finally he demonstrates the never ending ...

Check out this video to see our Fantastic Foamy Fountain in action. The experiment uses Hydrogen peroxide and dry yeast. Hydrogen peroxide is similar to water but has an extra oxygen atom. This makes it more dangerous, and only adults should handle the hydrogen peroxide.

Keeping things clean is very important to prevent food born illnesses. Washing fruit is important. You can take care of many problems by just running them under water. You can use white vinegar and hydrogen peroxide to clean fruit. Start by spraying some white vinegar on the fruit and then a little hydrogen peroxide. After you spray the fruit with white vinegar and hydrogen peroxide rinse it in water and that will wash all the vinegar and peroxide off of the fruit. The vinegar and peroxide wi...

Having less-than-brilliant teeth doesn't have to mean a trip to the dentist or a whitening kit. Try this all-natural remedy instead. Watch this video to learn how to whiten teeth naturally.

If you want to know how to clean your computer keyboard in 3 simple steps, you should watch this video. To clean your computer keyboard, you will need Hydrogen peroxide, soap dish, spoon, cotton buds, and a small bowl. Just follow these 3 simple steps: Put 1 part soap dish and 1 part Hydrogen peroxide into a small bowl depending on how much you need. Mix it well with a spoon. Dip the tip of the cotton bud into the mixture and scrub it against your dirty computer keyboard. Remember to change t...

Learn how to make a self inflating, flying condom! We should you how to make an instant flying condom using a few household items. Fantastic and fun science experiment! You will need one condom, one bag of hydrogen peroxide and dishwasher limescale remover.

Watch this instructional science video to learn how to create hydrogen from water, salt and electricity, for only a few dollars. This is an experiment that produces explosive gases, involves electricity and water and a number of risks so please be careful. Not an experiment to be performed by idiots. Create exploding water with this educational tutorial.

Elephant toothpaste is the name given to the catalyzed decomposition reaction of 30% hydrogen peroxide that uses soap to collect the oxgen gas that is produced. It is a favorite of most students at chemistry shows.

It's a small but very real frustration: you want a chilled drink, but you open the freezer only to see nothing but empty ice trays. Fortunately, there's a simple way to make ice cubes quickly—use hot water. Yup, you read that correctly. Hot water freezes more rapidly than cold.

I own two aprons—a cute one for company, and another for the hard-core cooking duties, like cutting up chicken and making stock. The sad truth is that I almost never remember to wear either of them. So, much of my clothing ends up spattered with grease, liquid, and bits of fruit and vegetable. While stain-removing sprays, sticks, and pens are all effective to a certain extent, they have two drawbacks—they're expensive and sometimes I need to use them in large quantity, like when a piece of eg...

Making a Elephant Toothpaste Volcano!

Looking to give your kitchen a good cleaning this spring? Don't go out and spend a ton of money on sanitizing sprays and cleaning equipment. Cleaning and sanitizing your kitchen is easy, effective, and inexpensive, because everything you need is likely in your kitchen!

Whether you're hanging out at the beach, laying by the pool, or walking around in sandals, having nail fungus is not the most attractive thing to flaunt.
Strawberries contain malic acid, which is great for removing stains on your teeth. You Will Need

Learn how to remove stains from wood furniture. Make your wood furniture look like new again by getting out rings and spots.

This video tutorial is in the Education category which will show you how to understand chemistry and chemical change. The question here is 10g of Mg ribbon reacts with 0.15 mol decimeter cubed of hydrochloric acid at 25 degree Celsius. What is the balanced equation for this reaction? When magnesium reacts with hydrochloric acid, you will get magnesium chloride and hydrogen. So, the equation will be Mg + 2HCl = MgCl2 + H2. Mg is in group 2 of periodic table and has a valance of 2, whereas Cl i...

In this video tutorial, viewers learn how to do a sodium and water experiment. Sodium is a silver metal that is very reactive. When exposed oxygen in the air, an outer coding of sodium oxide will form. Simply drop a piece of sodium into a cup of water. When dropped in water, sodium reacts to form sodium hydroxide and hydrogen gas. The sodium will constant move around in the water. Sometimes the heated reaction will cause the nitrogen gas to ignite. Under the right condition, it may even cause...

This small but very powerful electrolyzer produces gas that increases the fuel mileage of your car. The elctrolyzer runs on baking soda (4 volts/cell) and produces hydrogen. Electrolyzers in general are machines that uses a direct electric current to drive a chemical reaction that would otherwise not occur on its own.

Earlier this year, NASA reported on findings that might point to water, and microbial life, on moons orbiting Jupiter and Saturn. Named Europa and Enceladus, those moons contain large oceans under their icy surfaces, which many speculate could hold microbial life.

Attention aspiring scientists and chemists! Learning to balance equations and finding it to be a bit rough? This video is here to help. Learn the basics of chemistry with help from this video on how to balance equations with C (Carbon), H (Hydrogen), and O2 (Oxygen).
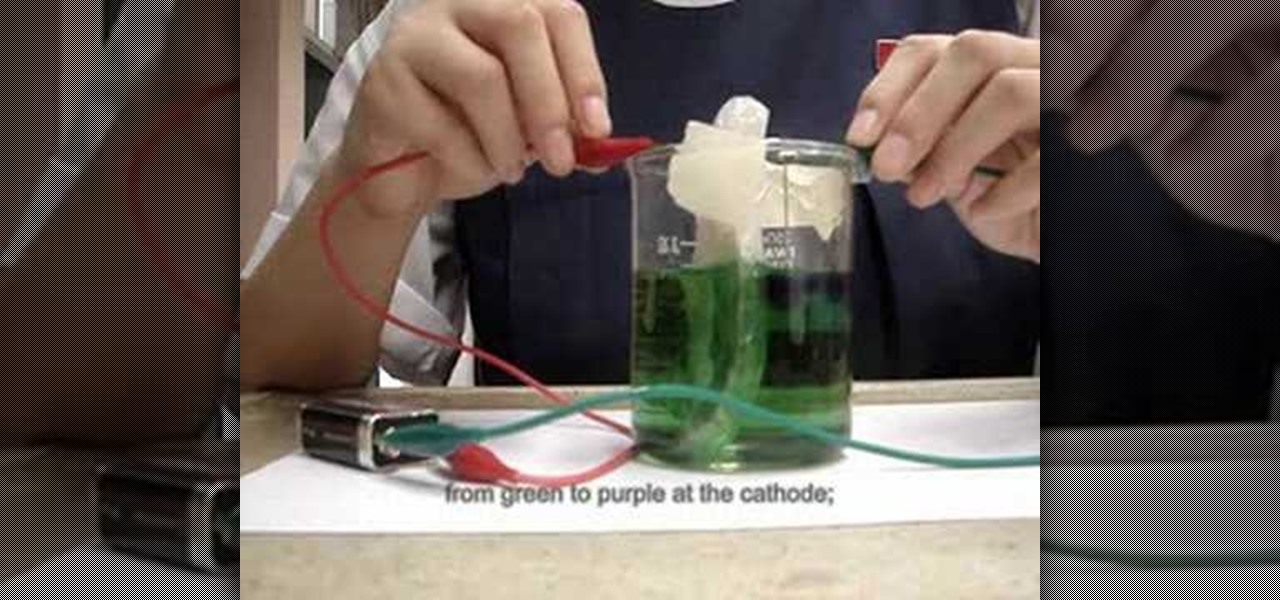
No, colorful electrolysis has got nothing to do with zapping the hair off of a punk rocker's head. Electrolysis of water, according to Wikipedia, is "the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water." In this video, you'll watch in amazement as a young scientist colorful electrolysis to transform ordinary water into a psychedelic display.

Learn how to light some hydrogen oxygen gas in the top of a milk jug with some aquarium hose run to it in order to create a small bolt of lightning.

See the electrolysis of water (baking soda dissolved) with copper wire. The negative side has hydrogen bubble, and the positive side has oxygen bubble. Watch the positive side as the water turns blue.

Start a fire using a flashlight a aloevera, hydrogen peroxide, and a magnifying glass. Super Easy!

Learn how to use electrolysis - the passing of an electric current through water - to get pure hydrogen and oxygen out of water.

Chemguy AKA Rob reviews the various diagrams in organic chemistry. This is a 16 part series from this Canadian high school Chemistry teacher.