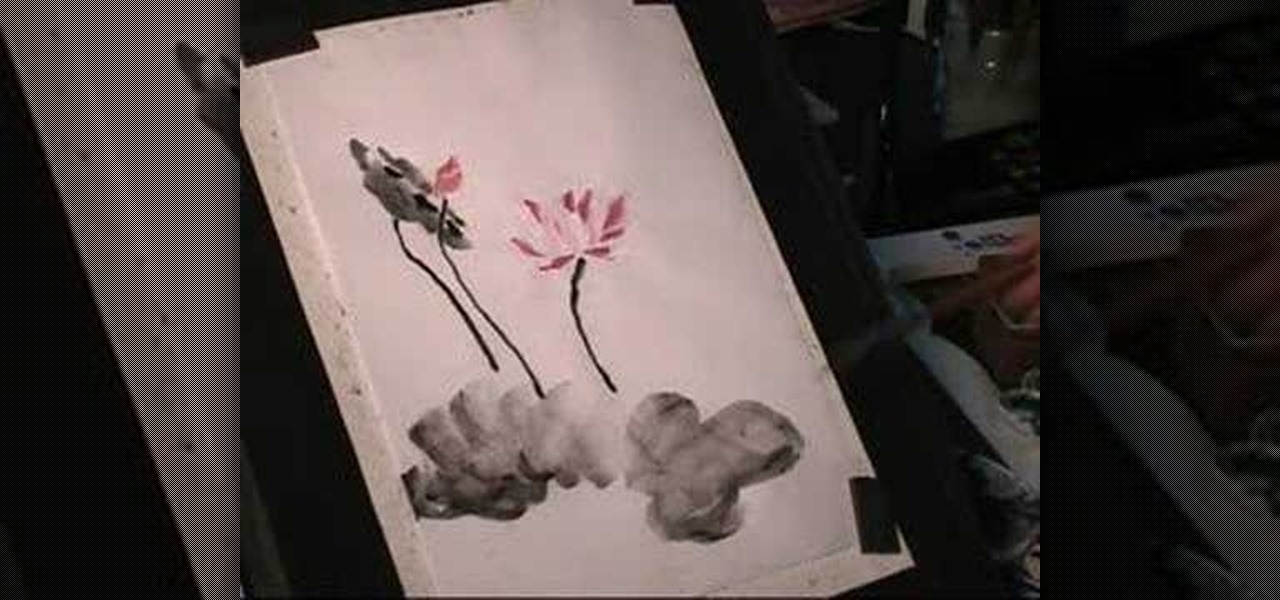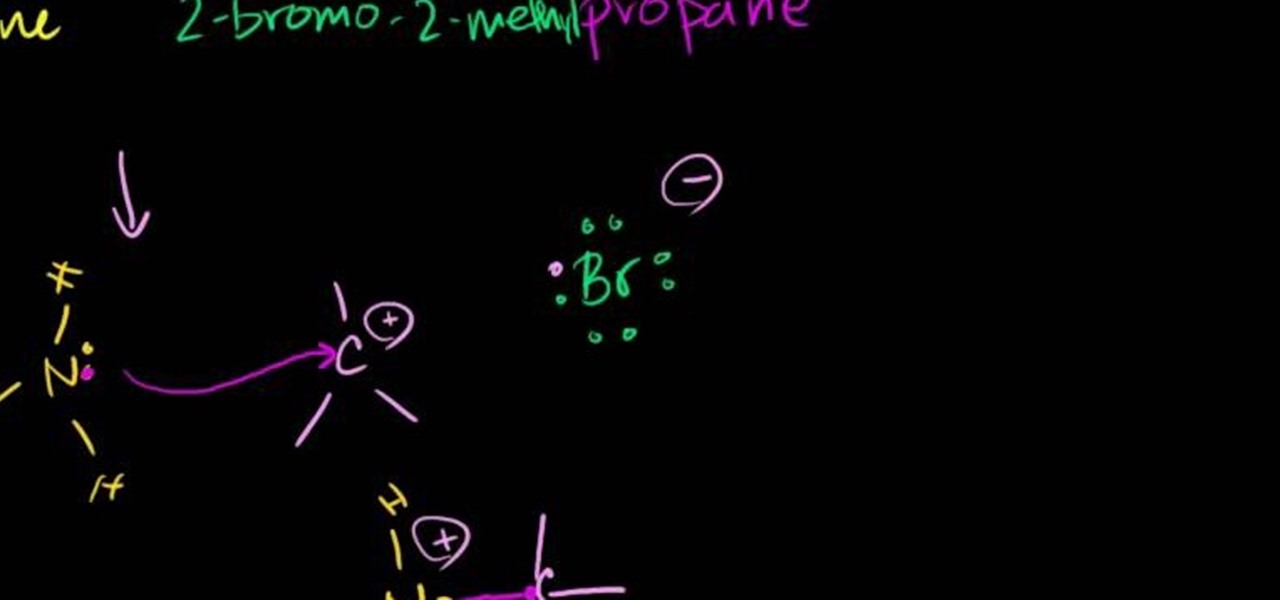There isn't a kid out there who doesn't enjoy gobbling up sugar cookies - unless he/she is allergic to gluten, that is. Gluten is a wheat product that some kids and adults have adverse reactions to, but the ingredient is included in many baked goods, from cupcakes to bread.

Chemistry can be very fun when you create different kinds of reactions between elements. Note that caution should be employed to not harm yourself while performing these reactions. Ammonium Tricynate reacts with barium hydroxide octahydrate in endothermic form. Endothermic reactions are those which absorb heat from the surroundings during the reactions as opposed to exothermic reactions which produce a lot of heat during the reaction. When endothermic reaction occurs, depending up on the inte...
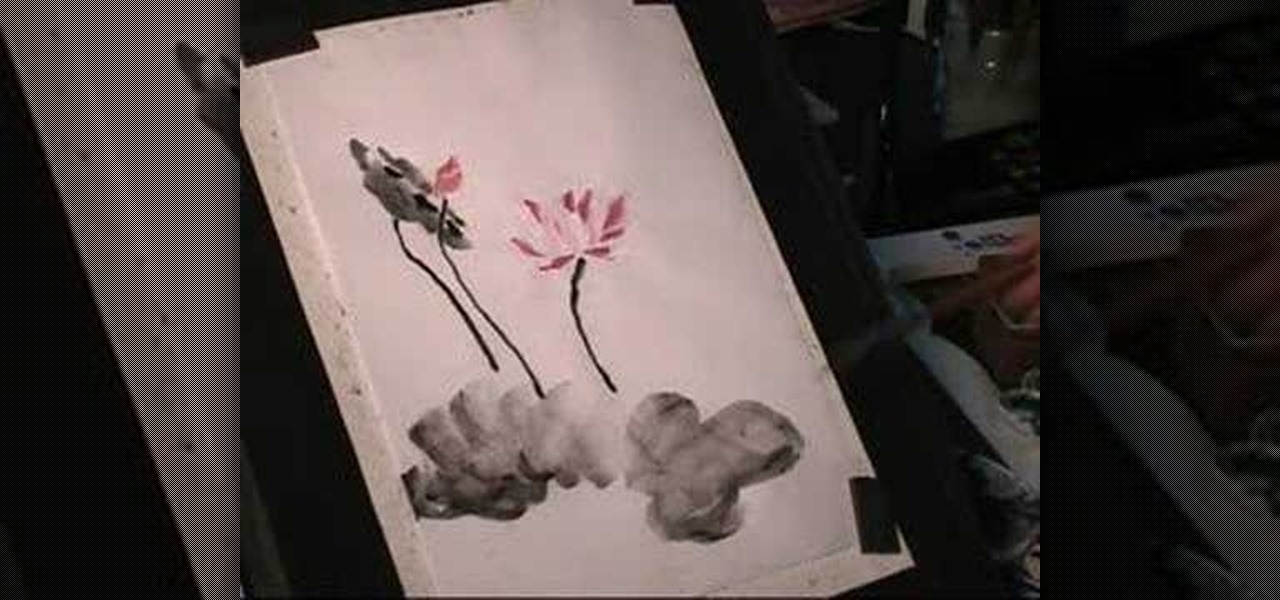
Chinese tradition has always honored lotus flowers. Essentially, the lotus flower represents peace, creativity and purity amid adverse surroundings. This is a perfect subject matter for sumi ink painting. Watch this instructional painting video to practice painting lotus flowers with ink and watercolors.

How can you quit smoking? Deepak Chopra details step-by-step instructions for curbing smoking habits in the long-term, which include feeling the adverse sensations of smoking and listening to your body as it rejects those sensations. Watch this video tutorial and learn how to quit smoking.

This video helps us understand the organic molecules and elimination reaction. Take some sugar in a beaker. Sugar has 12 carbon atoms, 22 hydrogen atoms and 11 atoms of oxygen. The sulphuric acid is poured into the sugar and the color change is observed. The color of the sugar gradually changes into black. The sulphuric acid causes an exothermic reaction which releases a large amount of sulphur dioxide gas. All the water (containing hydrogen and oxygen atoms) is eliminated out of the sugar du...

A pilot that earns an instrument rating is a pilot that's mastered his or her flight skills to a level or precision and accuracy needed to safely fly an airplane through clouds, fog, and other adverse weather conditions. While flying in these weather conditions, known as IMC, or instrument meteorological conditions, a pilot is tasked with flying an airplane solely by reference to flight instruments. The pilot needs to be able to go from takeoff to landing, without having any outside visual re...

Glowing substances have always held a powerful appeal to people, and making new ones can be a lucrative business. If you need some glow powder for a project of yours, watch this video to learn how to make DIY glow-in-the-dark powder out of normal household chemicals.

Watch this science video tutorial from Nurd Rage on how to dissolve glass with drain cleaner. They show you how to dissolve that glass with sodium hydroxide (drain cleaner).

Watch this science video tutorial from Nurd Rage on how to make a chemiluminescent reaction with home chemicals. Make a chemiluminescent singlet oxygen red light pulse from two simple chemicals almost anyone can buy: pool chlorine and hydrogen peroxide.

Acne sucks - or, more correctly, acne oozes. Which is why it's so important to take care of your skin with oil-reducing, calming products. Most anti-acne medications you can buy are severely drying and irritating, meaning they can cause the adverse effect of making your skin compensate by producing even more oil.

In this free video chemistry lesson from Salman Khan, we learn about SN1 reactions. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For all of the details, take a look.

A video which shows a reaction in which cobalt chloride reacts with water to form a hydrated form of cobalt and chloride ions.

In this video, I'll be demonstrating how anyone can make their own iodine clock reaction with simple over-the-counter chemicals.

Curious about reduction & oxidation? In this two-part episode of The Science Catalyst hosted by Barry Lambson, the subject of Chemistry, reduction & oxidation is gone over with iron powder and copper sulphate. Oxidation and reduction are heavily related which is why they are both considered "redox reactions". Acids and bases may be thought of as reactions involving hydrogen, or protons, while redox reactions tend to be concerned with electron gain and loss.

Watch this science video tutorial from Nurd Rage on how to make silver nitrate from silver and nitric acid. They show the chemistry of making this cool chemistry, colorless solid.

Watch this science video tutorial from Nurd Rage on how to make a glow stick reaction with real chemicals.

Making a Elephant Toothpaste Volcano!

Hydrazine sulfate has many uses, but most notably, it's been used under the trade name of Sehydrin, a treatment for anorexia, cachexia and some even think cancer. But for we DIY chemists, it's useful for something entirely different— as a substitute for the more dangerous pure liquid hydrazine in chemical reactions. NurdRage shows you how to make it via some hypochlorite and the Ketazine process.

C For Chemistry delves into the chemistry of science experiments. This chemist knows what he's talking about. These chemistry experiments are not only fun, but very educational for all of those interested in scientific chemical reactions and properties.

Geoff walks us through getting the "Chain Reaction" Achievement in GTAIV.

Elephant toothpaste is the name given to the catalyzed decomposition reaction of 30% hydrogen peroxide that uses soap to collect the oxgen gas that is produced. It is a favorite of most students at chemistry shows.

Have you ever run across a Facebook post that you don't necessarily want to "Like," but you're not really passionate enough about to bother stringing together a couple words for a comment? Well, you're in luck, as Facebook added five new "Reactions" that let you do more than just like a post, and they're now live for everyone.

This reaction is between metallic magnesium and carbon dioxide. Magnesium reacts with oxygen in the air to form magnesium oxide, but when the only source of oxygen is from CO2 the reaction becomes much more energetic. The products are white magnesium oxide and black carbon.

A new study has found that up to half of people who think they have a penicillin "allergy" can still receive the drug, and other antibiotics with similar structures, without any negative reactions to the meds. Why? Because they're not really allergic, doctors say.

In this free video chemistry lesson from Salman Khan, we learn about amines and SN2 reactions. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For all of the details, take a look.

In this free video chemistry lesson from Salman Khan, we learn how to work with aldol reactions. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For all of the details, take a look.

In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to undertstand the effects of solvents on SN1 and SN2 reactions. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

This free video science lesson from Internet pedagogical superstar Salman Khan presents a general introduction to the concept of reaction mechanisms in organic chemistry. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

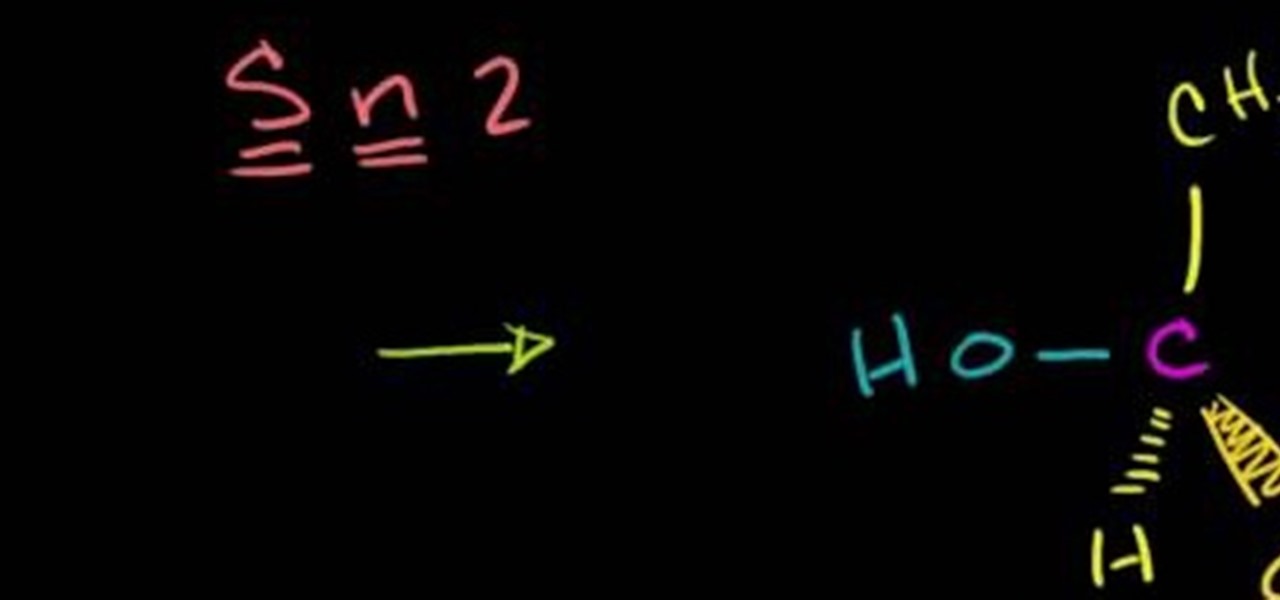
In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to understand and work with SN2 reactions in organic chemistry. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to handle SN2 substitution reactions in stereochemistry. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

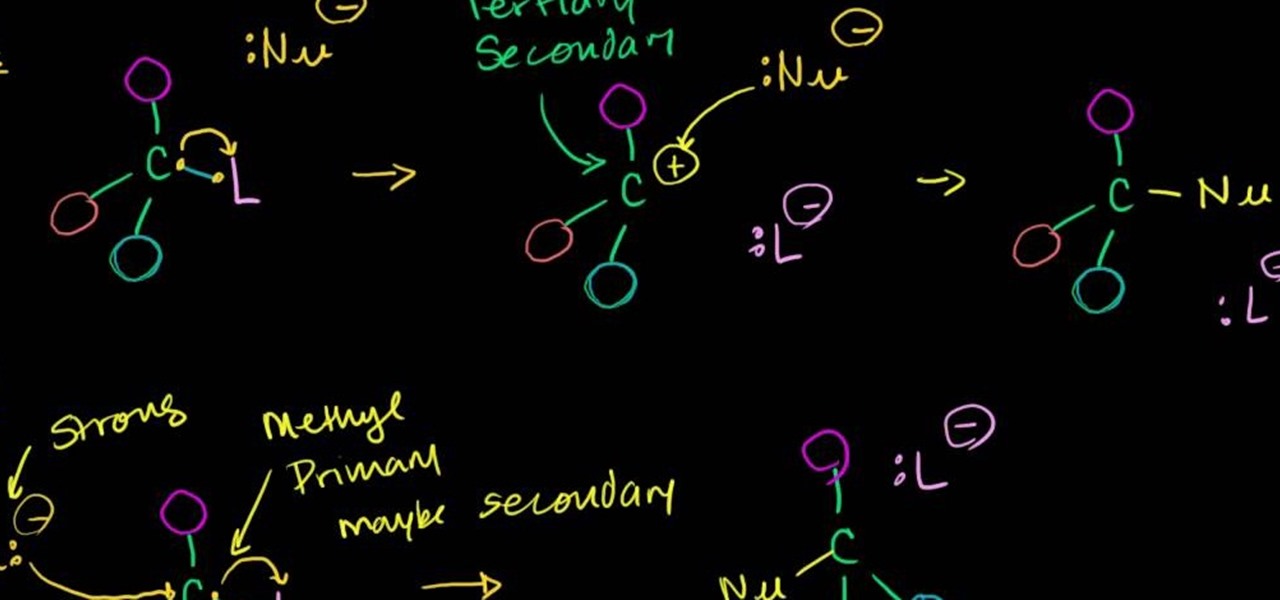
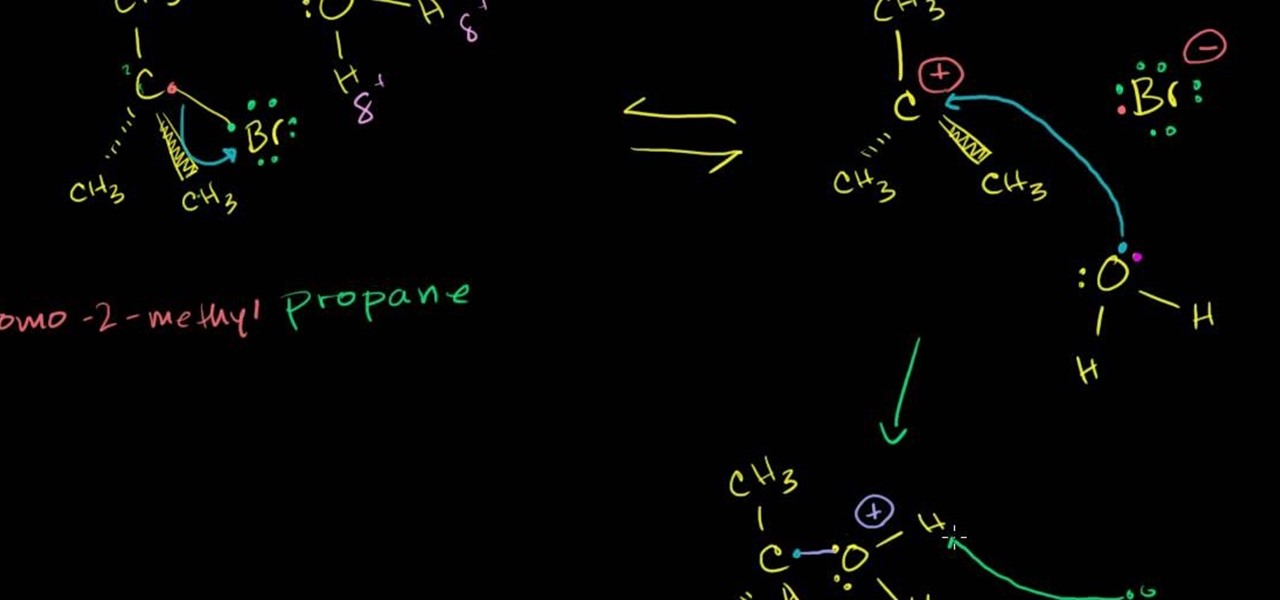
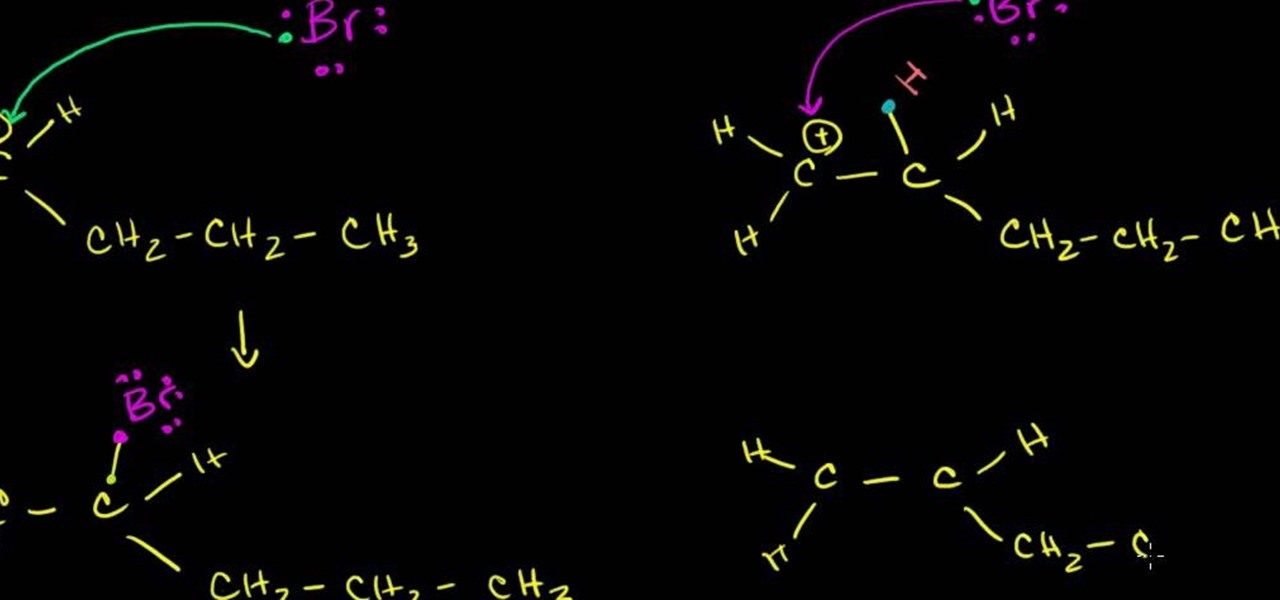
In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to handle SN1 reactions in organic chemistry. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to handle reaction mechanisms in organic chemistry. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

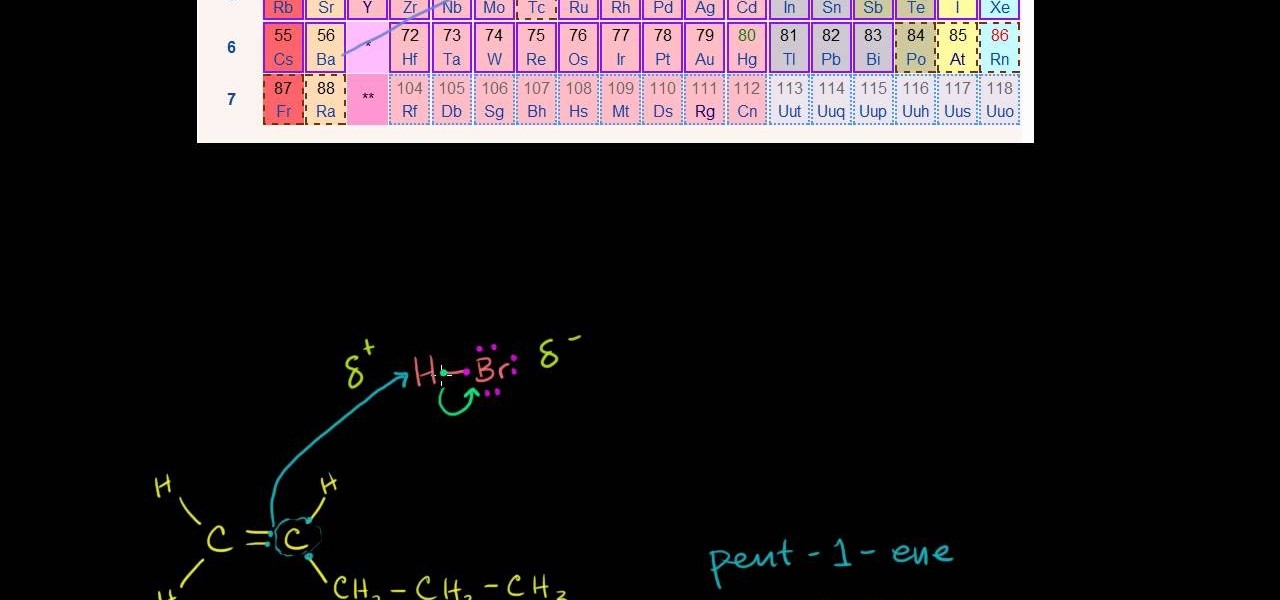
In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to use Markovnikov's rule to figure out which addition reaction is most likely in organic chemistry. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

In this video tutorial the instructor talks about Hydrochloric acid (HCL) and how it reacts to a few metals. To try this out take 30 ml of concentrated hydrochloric acid in a beaker. You need to employ caution while handling acids, especially if you use strong ones. Now you can throw small pieces of different metals into it carefully to see how it reacts with different metals. For instance when this HCL comes in contact with metals various reaction take place depending up on the metal. Like i...

This First Aid how-to video show you how to use an EpiPen in the event you or someone you know should experience an anaphylactic allergic reaction. Learn to save someone's life by learning to use an EpiPen properly.

Watch to see a demonstration of how a nuclear reaction works using a matrix of ping-pong balls set on top of mouse traps at a Physics show at the University of Bonn.

Check out this video to learn how to do the "chain reaction" yo-yo trick.

Once used as solid rocket fuel because the reaction requires no oxygen, sulfur and zinc react vigorously. The reaction with zinc produces flame and a near explosion. Sparks fly and smoke billows in this dramatic chemical demonstration.

This illustrates the reaction had between magnesium and dry ice. It ignites in a chemical reaction between the CO2 & the magnesium.

Once you've reached an age where sparklers are no longer fun, it's time to upgrade to science and steel wool. It may be basic chemistry, but as you'll see in the video, simplicity can amaze more than complexity, as well as create some really impressive fireworks that are perfect for the Fourth of July.