
How To: Sugar & Sulfuric Acid — A Cool Chemical Reaction
Sulfuric acid is mixed with sugar, which is attacked by the acid. The final products are carbon, water vapor, and sulfur dioxide gas.


Sulfuric acid is mixed with sugar, which is attacked by the acid. The final products are carbon, water vapor, and sulfur dioxide gas.

You already know how to make sulfuric acid with the metabisulfite and oxidizer method and you saw how to make copper sulfate from copper and sulfuric acid, so now try making sulfuric acid with these two in mind… with sulfuric acid by electrolysis of copper using an inert anode.

Watch this science video tutorial from Nurd Rage on how to make copper sulfate from copper and sulfuric acid in three ways. They show you how to make copper sulfate from copper and sulfuric acid using two chemical methods and one electrochemical method.

Watch this science video tutorial from Nurd Rage on how to make iodine from sulfuric acid and alkali metal iodide. This is the best way to make elemental iodine from sulfuric acid and sodium or potassium iodide.

In this home scientist video the instructor Robert Bruce talks about cheap sulfuric acid. He says that sulfuric acid is very important in any lab both as a reagent and a precursor for preparing other chemicals. He points to the battery acid saying that it is a good source of sulfuric acid which is 35% concentrated. Now he shows various methods to obtained sulfuric acid and shows how to test one of the thus obtained sulfuric acid for its concentration. In this video the author talks about sulf...
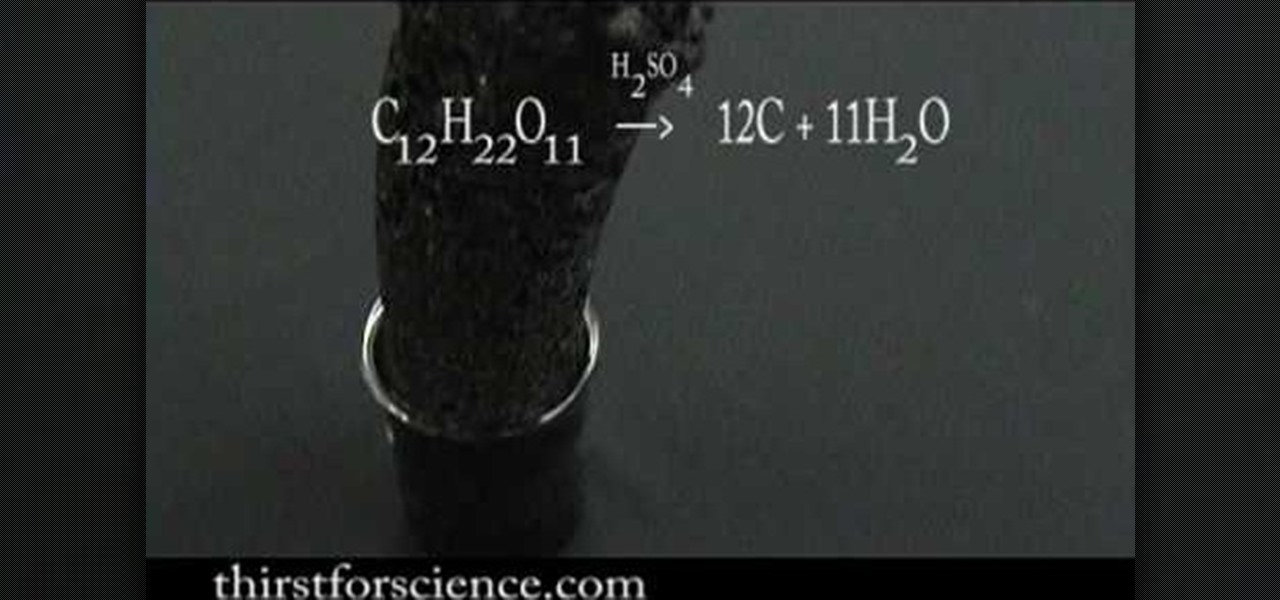
Sugar (a carbohydrate) is dehydrated with concentrated sulfuric acid. Since a carbohydrate was once considered just hydrated carbon, if you remove the water, carbon would be left over. The acid rips the water out of the sugar and the heat generated by this reaction causes the water to turn to steam. A black mass of carbon is produced.

This video is based on a chemistry experiment. This video is going to explain to us how to use gravimetric analysis in order to analyze sulfuric acid. This experiment should not be done for those who do not have a fundamental knowledge of chemical compounds, reactions and chemical safety. He explains first what he is about to do before he does it. Sulfuric acid is acidic and can be toxic if ingested or in contact with the eyes. Take extreme care with this experiment.

Sugar (a carbohydrate) is dehydrated with concentrated sulfuric acid. Since a carbohydrate was once considered just hydrated carbon, if you remove the water, carbon would be left over. The acid rips the water out of the sugar and the heat generated by this reaction causes the water to turn to steam. A black mass of carbon is produced.

Watch this science video tutorial from Nurd Rage on how to make a test tube thunderstorm. They show you how to make the thunderstorm in a test tube using alcohol, sulfuric acid and potassium permanganate.

Watch this science video tutorial from Nurd Rage on how to make fire 4 ways without matches by using chemistry, without matches or lighters.

What is MnSO4 and MNO2, anyway? They are they molecular formula for Manganese Sulfate and Manganese Dioxide. And you can make one from the other. But how?

Process of making Hydrochloric acid using table salt and concentrated sulfuric acid. Consentration of the syntethised acid was 2.7M or 10%. Music: 1200 Mics - Rock into the future.

Watch this science video tutorial from Nurd Rage on how to make iodine from an alkali metal iodide, hydrochloric acid (HCI), and hydroxide peroxide (H2O2).

Watch this science video tutorial from Nurd Rage on how to make nitric acid. They show three ways to make nitric acid based on two different chemical approaches, both of which can be done using easily accessible materials.

Most seeds have a thick outer shell meant to protect the soft inner seed. 'Nicking' is a gardening technique to remove the outer shell so the seed will germinate faster in your garden after planting. You can use water, sandpaper, a nail file or even sulfuric acid to nick your seeds.

C For Chemistry delves into the chemistry of science experiments. This chemist knows what he's talking about. These chemistry experiments are not only fun, but very educational for all of those interested in scientific chemical reactions and properties.

C For Chemistry delves into the chemistry of science experiments. This chemist knows what he's talking about. These chemistry experiments are not only fun, but very educational for all of those interested in scientific chemical reactions and properties.

In this video, I'll be showing you how classic black snakes work and how to make them at home. There are actually two methods covered in the video — one that uses fire and one that does not. So just choose the one that fits best for your situation.

Want to make boring old colorless water brighten up on command? Well, you can control the color of water with this little magic trick. Actually, it's not really magic, but a classic science experiment known commonly as the iodine clock reaction, which uses the reactions between water and chemicals to instantly colorize water, seemingly by command. You can use different colorless chemicals to produce different colors, and you can even make the color vanish to make the water clear again.

In this tutorial, we learn how to make hydrochloric acid from salt. First, you will pour some salt into a distil flask. After this, you will add in some concentrated sulfuric acid to the salt. Next, you will let these react with each other. You will start to see gasses bubble up and the excess hydrogen chloride gas come out through the top of the tube. To create a stronger reaction, you can add heat underneath the reaction. Then, test this by exposing it to ammonium chloride. If it's the righ...

We've shown you how to make water change color on command, but how about just half of it? What if I told you that you can split a solution right down the middle and make the color disappear from one side, just by shining light on it?
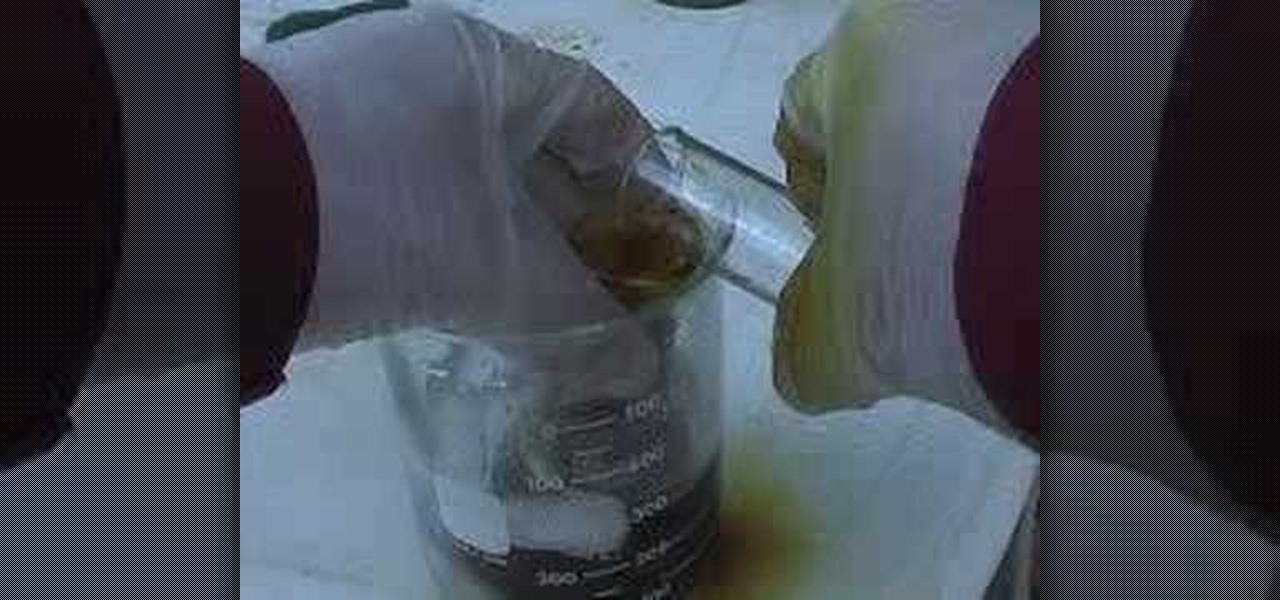
In this video, we learn how to make iodine easily. You will need potassium iodine and sulfuric acid to make this. First, add the acid into the potassium iodine slowly. After you add in each part, swirl the beaker slowly so it gets mixed together. After you have added in all of the potassium, you will place this into a beaker filled with ice water while you add in more, because the mixture gets really hot. When finished, you will end up with a mixture that is iodine and nothing else. Fill with...

This science experiment will show you how to make a storm inside a test tube. This video tutorial will demonstrate the process of making the miniature thunderstorm inside a test tube with just a few common chemicals. All you need for your very own thunder storm is a glass test tube with holder, sulfuric acid, ethyl alcohol (ethanol), potassium permanganate, glass dropper, measuring spoon, and please wear safety goggles. Sparks and pops occur completely random, just like in a real thunderstorm!

A fire snake, also referred to as a black snake or sugar snake, is a classic science experiment you can do right in your own kitchen using a baking soda and sugar mixture and a fuel to ignite the reaction.

Hydrazine sulfate has many uses, but most notably, it's been used under the trade name of Sehydrin, a treatment for anorexia, cachexia and some even think cancer. But for we DIY chemists, it's useful for something entirely different— as a substitute for the more dangerous pure liquid hydrazine in chemical reactions. NurdRage shows you how to make it via some hypochlorite and the Ketazine process.

Various electrochemical reactions requires that anodes do not degrade when used. Carbon is cheap, but degrades easily and platinum is extremely expensive. In a previous video, you learned "How to make cobalt and manganese nitrates", and you saw that titanium could be used as a cathode, but not as an anode due to an effect called passivation.

This video speaks to everyone who has ever bought anything online, or in fact, anyone who has ever bought anything period. How do you know what you're getting is genuine? Is it a fake product? Is it stolen goods? Is it impure?

Watch this science video tutorial from Nurd Rage on how to make a desiccator bag for drying chemicals with Dr. Lithium.

Chlorine gas is a very useful oxidant, which was first introduced as a toxic weapon by the German Army. Even today, it's still used as a weapon, most recently in the Iraq War by insurgents. But chlorine gas has more useful (and less lethal) applications, and if you want to learn how to make some at home, NurdRage has the answers.

We know that "acid green" is not exactly a delightful color when it comes to most things. If your two month-old orange is acid green, it's probably pure mold, and if a bottle is full of acid green liquid, it's most likely poison (or, okay, soda). But you get our point.

Aqua regia (königswasser in German) is a very corrosive liquid made from a mixture of nitric acid and hydrochloric acid (1:2 - 1:3). This chemical mixture is so corrosive that it can even dissolve gold, and that's what you'll learn about in this video.

This three-part video tutorial demonstrates how to acid wash and paint a swimming pool. In part 1, host Tim Casey shows you how to properly and safely acid wash your swimming pool to prepare for painting. Part 2 discusses the steps involved in repairing cracks in a pool wall, step and deck while Part 3 covers the actual pool painting process.

This is a video tutorial in the Education category where you are going to learn how to make boric acid from borax. For this experiment you will need borax (disodium tetra borate) and conc. hydrochloric acid. Take 25 ml of hydrochloric acid and dilute it with 75 ml of water. Next take 6 - 7 gms of borax and dissolve it in boiling water. Now add equal amount of hydrochloric acid. Crystals of boric acid will start forming. They are completely insoluble in cold water. After about half an hour, fi...

In this free video chemistry lesson from Salman Khan, we learn how to use acid-base titration to find the mass of oxalic acid. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.

Aluminum nitrate nonahydrate is a crystalline hydrate - a salt of aluminum and nitric acid - Al(NO3)3·9H2O. It's used for a variety of things such as antiperspirants, corrosion inhibitors, and petroleum refining, or… glow-in-the-dark powder. Watch this science video tutorial from Nurd Rage on how to make aluminum nitrate nonahydrate with Dr. Lithium.

This is chemistry at its best! Europium is the chemical element (Eu) which was named after Europe. Dysprosium (Dy) is a rare earth element of a metallic silver luster. Watch this science video tutorial from Nurd Rage on how to make europium and dysprosium nitrate salts with Dr. Lithium.

Relieve symptoms of indigestion and hear burn with some simple remedies. You Will Need:

This two part video goes over the procedure for testing and inspecting lead acid batteries. A machine is required to test the battery itself. You can do the visual test at home, and then bring it to an auto parts store for the machine test part.

Acid reflux can be painful and irritating, and did you know that toddlers can be affected by it too?

This video topic was changed. It is now converting muriatic acid to reagent grade hydrochloric acid, HCL).